Lab Service
At Ions Lab, our goal is to make sure your individual testing profile meets both regulatory compliance and your overall program objectives in the most accurate and timely manner possible. Our technical and customer service staff will work with you to choose the appropriate tests, methods and frequencies best suited to your specific needs. Whether it’s raw materials, the production environment or finished products, our goal is to partner with you to mitigate risks wherever they occur in your supply chain.
From routine analytical testing to special projects, in the US or around the World, Ions Lab is your best choice for a Laboratory partner in today’s complex scientific and regulatory environment. Feel free to contact one of our representatives if you have questions or if you need more information on other microbiology testing.
Examples of process control, quality and spoilage tests we perform include:
| Aerobic Plate Count YeastMold | Total Coliforms and E. coli Anaerobic Plate Counts Mesophillic and Thermophillic Sporeformers | Lactic Acid Bacteria Alicyclobacillus |
Examples of the pathogen tests we perform include:
| Salmonella Campylobacter jejuni/coli/lari Listeria monocytogenes Enterobacter sakazakii (Cronobacter) | Listeria species E. coli O157:H7 Staphylococcus aureus Clostridium perfringens | Bacillus cereus Shigella Vibrio cholera/parahaemolyticus/vulnificus |
In addition to the routine Testing, Ions Lab offers specialized services which may be tailored for each individual customer. Special Studies include:
- Shelf Life Studies
- Spoilage Investigations in Canned Foods
- FDA Detention Related Testing – Salmonella, Listeria monocytogenes, etc.
- Challenge Studies
- Environmental Monitoring Sampling Programs
It is our belief that in order to get quality products quickly, our quality control lab and testing procedures should be able to process materials in an efficient and thorough manner. Therefore, we run almost all of our raw material, finished product and stability testing in-house using fully validated methods on qualified and certified equipment, using trained chemists and biologists.
Our lab processes and assesses each raw material, finished product, and stability product that comes through our doors and is produced at our facility. In order to perform this daunting task, we need the highest quality personnel and equipment.
Quality Control Lab and Testing Equipment
Chromatography – United States Pharmacopeia depicts chromatography as a procedure where complex mixtures can be separated and quantified using their specific properties. This is a powerful tool in assessing identity, purity, and strength from pure raw materials to complex finished products.
– HPLC/Mass Spectroscopy or High-performance liquid chromatography is one of the best ways to quantify an analyte. All methods used at ion are fully validated to assess precision, accuracy, and linearity to ensure that analytes tested can be accurately quantified in our products. Furthermore, when applicable, we only use USP traceable standards to give us the highest precision possible when quantifying even micronutrients. Our lab also possesses cooled autosampler technology allowing us to refrigerate samples while testing, slowing the sample degradation process for unstable analytes. Finally, we have a single quadrupole mass spectrometer, which we have used in our more complex validations.
-TLC or thin layer chromatography is based on similar science as HPLC. This technique is a powerful tool in identifying actives in complex botanical mixtures. All of our standards are high quality fully characterized materials from highly reputable vendors like ChromaDex.
-AA or atomic absorption is used to identify and quantify minerals and metals in raw material and finished products. Using fully validated methods for each mineral and metal tested, we can assure minerals added meet label requirements and confirm that raw materials and finished products are within safe limits for heavy metals.
-Microbiology. Ion has a fully equipped micro station, which includes: incubators, isolated pipets, autoclave, and HEPA-filter micro hood. This allows us to accurately assess the quality of our products using USP methods that have been verified in-house. We routinely test for Salmonella, E. coli, S. aureus, standard and virulent yeast and molds, Pseudomonas strains, and total aerobic counts.
– UV Spectroscopy is used at Ion Labs to identify and quantify the proteins used in our products. Using a fully validated procedure and traceable standards, all required raw materials and finished products are run using a modified protein Lowry procedure.
To constantly provide customers with the best quality, we are constantly working to adhere to the standards set forth by the FDA, NSF, USDA and other highly reputable governing bodies. We are constantly improving and enlarging our technologies, methods, and personnel to make sure we have the best in the industry.
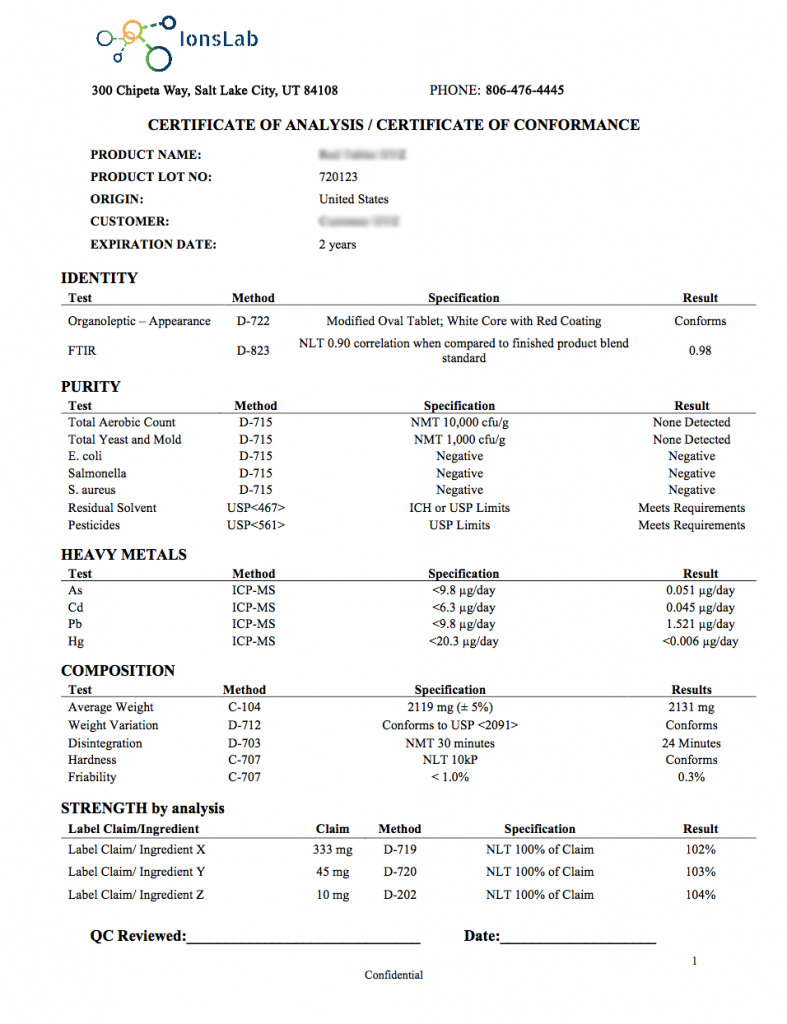
Below is an example of an Ions Lab Certificate of Analysis: